Commissioning, Qualification & Validation: Fundamental for pharmaceutical products & life-saving therapies
Operating a GxP facility that must comply with regulatory requirements is no simple matter but doing so ensures that products are manufactured and delivered safely under meticulous regulation and standards. Regulations require that manufacturers in healthcare industries, pharmaceutical companies, and small laboratories use equipment, systems and processes that are up-to-date and fit for purpose. Applications range from discovery to development, manufacturing, packaging, and labeling, receiving, storage, to testing and quality control activities. Here is where design-focused architecture and engineering, along with commissioning, qualification, and validation (CQV), become key to compliance with Current Good Manufacturing Practice (CGMP).
Facilities that will be used for activities regulated according to CGMP should be designed for CGMP. Along with design and construction, GMP-compliant facilities benefit from commissioning, qualification, and validation (CQV). CQV services can include design qualification (DQ), installation qualification (IQ), operation qualification (OQ), and performance qualification (PQ). In addition to its design-build capabilities, Genesis AEC provides a comprehensive set of CQV services to ensure life science firms can efficiently start-up, maintain, or renovate their facility, utilities, systems, and equipment through risk-based commissioning and qualification.
Genesis AEC is an award-winning firm that provides consulting, architecture, process engineering, construction management, as well as commissioning, qualification, and validation to the life science industry. For over 25 years, Genesis AEC has provided AE support along with EPCMV (Engineering, Procurement, and Construction Management, CQV) services. Services can include early preplanning during design through construction, execution, and turnover, and mechanical, electrical, and plumbing (MEP), and Fire Protection and Process Utility.
Expert at providing expertise

Nathan Roman is Director of Validation at Genesis AEC, responsible for Qualification and Validation consulting services. He has over 21 years experience in commissioning, qualification, and regulatory compliance services and is an expert in CQV and CGMP compliance.
“The scope of what Genesis AEC provides is always dependent upon the client’s needs,” says Roman. “First, gaining an understanding of what you are doing and how you plan to do it; getting the teams on the same page with where we are going and how we are getting there. Typically, we put together a plan, set expectations, discuss desired outcomes (describing what success will look like), build the schedule, and then execute. The plan aligns goals, objectives, and a vision of the project to ensure that both Genesis AEC and the client are on the same page.
“One of our main service offerings is Commissioning, Qualification and Validation. A typical comment I hear from customers is, ‘You’ve seen what’s going on in industry. We need that expertise.’ So along with project work and deliverables, we share the knowledge we’ve gained having worked for many companies and on many types of equipment and systems within GxP-regulated industry. Additionally, our clients’ projects often benefit from our flexible staffing solutions or temporary added human resources, which come pre-equipped with the relevant expertise required. This ensures our clients’ staff can stay focused on operations and scale up as needed.”
Genesis AEC works closely with their health and life science client firms, often providing staff augmentation. “A common scenario is that the client will have goals with a timeline that necessitates added human resources,” says Roman. “We can provide a number of skilled and qualified consultants, say validation engineers, with the level of experience that fits the project needs. This creates added value and builds long-term relationships.”
“If we find that we are attempting to qualify equipment that isn’t going to effectively achieve the end goal of the customer, it’s our job to let them know there’s a better way. We don’t blindly qualify or validate. We look at a process or system based on its objective. Then we guide our clients to better solutions.” Nathan Roman, Director Validation Services, Genesis AEC
Efficient, effective compliance in life science industries, regulations are well-known. The questions come from finding the best way to comply. “When designing, building, and qualifying for GxP, you can find ways to be efficient, including risk analyses, identifying the critical design controls, and leveraging tests that have already been completed during engineering start-up and commissioning. This saves time and cost, without sacrificing the goals of the qualification process,” says Roman.
“Everybody knows what needs to be done, but they’re not always sure how to get there. We definitely know how to get there because that’s what we do, over and over again. We do things in ways that have been proven to be effective, says Roman. “Our work should always align with a firm’s existing quality system. Sometimes we find a very conservative approach to validation based on how people are accustomed to seeing documents. But we still try to help the client find efficiencies wherever possible.
“Regulatory enforcement seems to have become stricter over the past few years because of non-stop technological advances, data integrity requirements, and the intense focus on vaccine production and distribution. Many companies reach out to us seeking help with addressing observations that have resulted from an inspection. We review the observances and work with the client to create a remediation plan,” says Roman.
“For corrective actions, we often see companies put too much pressure on themselves by responding to the agency with a super-aggressive timeline, having committed to address observances as quickly as possible. But we can help them with this too because we know the FDA expects that you are actively following your CAPA process and implementing remediation with an appropriate amount of time to correct and prevent the issues from happening again. You can provide updates on a periodic basis and revise your timeline to be more realistic and complete.
“Increasingly, the FDA is looking closely at the qualification of the systems, equipment, and instruments. They scrutinize data integrity, for example noting time and date stamps on records, and they expect access controls on computerized systems.”
Roman recalls a recent experience qualifying multiple applications in a laboratory seeking to show compliance with Good Laboratory Practice (GLP). “The company was US-based, with an additional site in the UK, so the inspecting agency was the MHRA. In this situation they needed all compliance documentation, SOPs, risk assessments, calibration procedures, qualification protocols, computerized system validation protocols, periodic review procedures, etc. We assisted them every step of the way to obtain MHRA approval and gain their initial GLP certification in UK. We created a plan, set expectations, discussed the desired outcomes, put a schedule in place, and executed the plan. After we had completed the qualification and validation, they successfully passed their inspection. It’s always gratifying to see our clients succeed.”
Knowledge sharing for long-term success
Along with consulting services, Genesis AEC trains their clients on best practices, especially for Good Manufacturing Practice (GMP). Roman says, “When we leave a project, the staff not only have compliant documentation, but also a better understanding on how that compliance is achieved. We give a top-down view of why we design, build, or qualify the way we do so that they understand the principles. When you’ve only ever done something a certain way at Company A, you might not spot inefficiencies or risks to compliance or quality. It is beneficial, especially prior to a regulatory inspection or survey, to hire a company with broad experience at Companies B, C, and D, etc. Then you have the added perspective of a consultant who can give you a simpler, more effective way of doing things.”
Not all of guidance happens in response to enforcement action. Many firms find it’s more efficient to have the consulting work done prior to inspection. “We work with our clients to provide a compliance review of all their qualification documents, pinpointing areas that need work,” says Roman. “Pre-inspection reviews can help the company feel more confident anticipating an unannounced visit from a regulator.”
Validation: Proving the process ensures the end-product
As the Validation Director of Genesis AEC, Roman provides leadership in validation and qualification for new equipment and instruments, as well as computerized systems. His work ensures compliance with CGMP and other internal and external quality and regulatory guidance. With over twenty years of experience, he’s seen many changes over time.
“In terms of what is required for compliance with CGMP, little has changed,” says Roman. “However, in regard to guidance documents much has changed over the last decade or so. Over the years, the roles and responsibilities of commissioning engineers and validation personnel have changed. From the introduction of the V Model to the first edition of the ISPE’s Commissioning and Qualification Baseline Guide, to the more recent Quality Risk Management (QRM) life-cycle approach.”
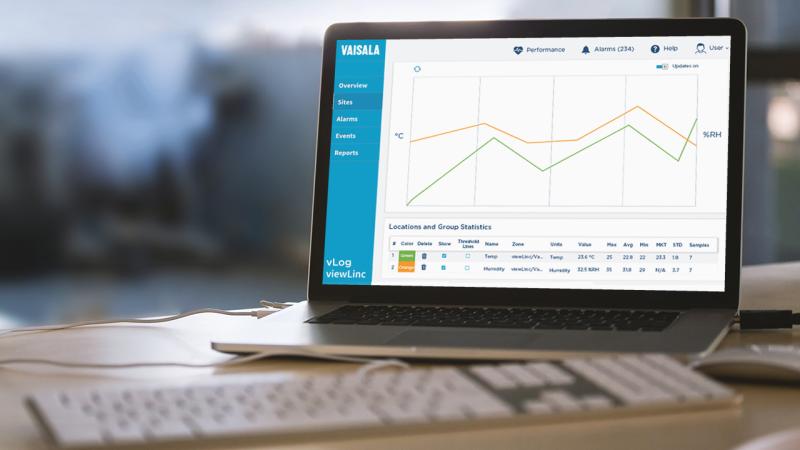
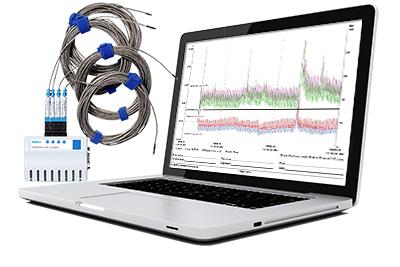
Another change over the years has been the equipment used in mapping studies to qualify storage areas. Increasingly firms are turning to data loggers. “Way back when, temperature mapping was all about thermocouples. With that equipment we needed to check our wires and sensor tips, fixing them if needed, then wire up the sensor module, set up our mapping study in the software, connect all the components for calibration purposes, run a calibration study; all this was done prior to even setting up a chamber for qualification. It was extremely time consuming, and the equipment was cumbersome,” says Roman. “Once I discovered the Vaisala data loggers with the vlog software, we saved a ton of time on mapping applications. The Vaisala system is very plug-and-play. The reporting features of vLog allows you to format data easily.
“To some, validation and qualification might seem like long, drawn-out processes, but there’s a good reason for it. If you can ensure your equipment or systems perform consistently and meet industry requirements, the value of both the product and patient safety increases.”
“It’s true that commissioning, qualification and validation is filled with documents upon documents, testing and more testing, with many hours spent in the field or behind your desk. But understanding how to be efficient in validation is an integral step to quality assurance and the many different aspects of compliance in life science industries. “The work we do matters. Helping life science organizations ensure quality and safety when carrying out commissioning, qualification, and validation projects in an efficient manner ultimately leads to saving lives.”