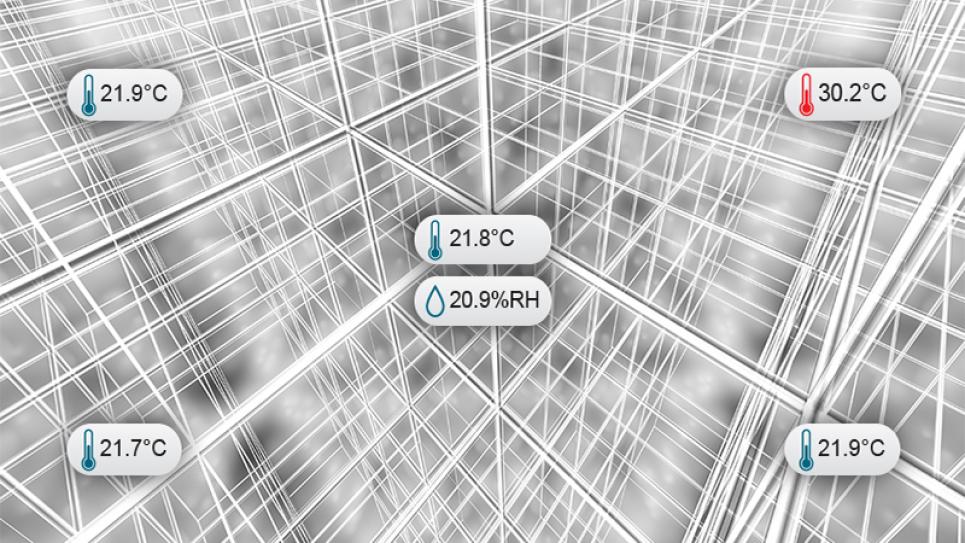
This learning module maps out each corner and everything in between when it comes to validation mapping studies. The Food and Drug Administration's (FDA) regulations tell us that we must identify the environmental conditions that can affect the strength, identity, safety, quality, and purity of our regulated products, whether they are pharmaceuticals, medical devices, or biological products. Get the job done right the first time with the following materials.
- Webinar: Mapping made easy, where to place sensors and why
- Application Note: 5 frequently asked questions on temperature & humidity mapping
- White Paper: Step-by-step guidelines for validating life science storage facilities
- Webinar: Continuous mapping for warehouses
- Application Note: Temperature mapping with viewlinc