Optimizing Ammonium Nitrate Production with Refractive Index Technology
Ammonium nitrate (NH4NO3) is a versatile salt composed of ammonium ions (NH4+) and nitrate ions (NO3-). It plays a crucial role in the explosives and fertilizers industries and is also used in the treatment of titanium ores.
The Ammonium Nitrate Production Process
Ammonium nitrate is produced by reacting nitric acid with ammonia. The resulting solution is concentrated to 97.5-98% in a final concentrator. This concentrated solution, along with quality additives, is then fed into a prilling tower where small pellets of ammonium nitrate are formed. Some of the concentrated solution is also gravity-fed into a slurry tank.
In the slurry tank, the filler is dispersed, and the oversize and small fines are melted. The 97.5% ammonium nitrate solution from the concentrator is discharged, and the moisture content of the mixture is adjusted by adding scrubbing liquor. The slurry is then conveyed through a hot air atomizing system to form tiny droplets, which are sprayed onto a curtain of falling granules from the prilling tower.
Ammonium Nitrate Application & End Products
The primary end products of ammonium nitrate production are fertilizers and explosives. The production process involves several stages, including the formation of pellets in the prilling tower, classification into fractions, and cooling in a fluidized bed cooler. The cooled ammonium nitrate is treated to prevent caking during storage, typically by spraying a liquid coating agent made of oil and amine in a rotary drum.
Process Refractometer Instrumentation & Installation
The Vaisala Polaris® Process Refractometer PR53GP is essential for continuous monitoring of the ammonium nitrate process. It is installed on the concentrator and slurry tank outflows to ensure a uniform prill and prevent the need for reprocessing. Traditional methods of calculating concentration based on nuclear density and paper charts are often confusing and time-consuming. In contrast, the refractometer provides a direct measurement of ammonium nitrate concentration, which can be sent to the control room via Ethernet or 4-20 mA output signals. This real-time data allows operators to make immediate adjustments to the process.
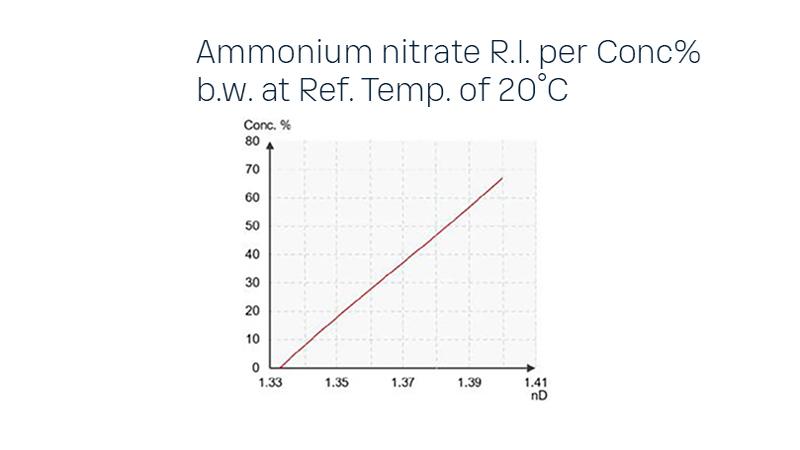
Refractive Index Measurement Range and Accuracy
The refractometer measures the refractive index (nD) in the range of 1.3200 – 1.5300, corresponding to 0-100% by weight. The concentration of the outflow NH4NO3 solution is typically 90-98%, with process temperatures ranging from 160-180ºC (320-356ºF). In the slurry tank, the NH4NO3 solution concentration is also 90-98%, with temperatures between 150-160ºC (302-320ºF). The typical measurement range is 60-100% NH4NO3, with an accuracy of 0.2% NH4NO3. An automatic prism wash with steam may be required for this application, and appropriate equipment with hazardous and intrinsic safety approvals is available when needed.
Optimizing ammonium nitrate production processes with refractive index technology ensures high-quality end products and efficient operations. The Vaisala Polaris® Process Refractometer PR53GP provides reliable, real-time measurements that are crucial for maintaining the desired concentration levels and making necessary adjustments promptly.
For more information, please contact us.



Add new comment